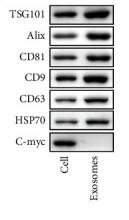
产品描述
*The optimal dilutions should be determined by the end user.
*Tips:
WB: 适用于变性蛋白样本的免疫印迹检测. IHC: 适用于组织样本的石蜡(IHC-p)或冰冻(IHC-f)切片样本的免疫组化/荧光检测. IF/ICC: 适用于细胞样本的荧光检测. ELISA(peptide): 适用于抗原肽的ELISA检测.
引用格式: Affinity Biosciences Cat# AF5466, RRID:AB_2837950.
展开/折叠
DnaK type molecular chaperone HSP70 1; Epididymis secretory protein Li 103; FLJ54303; FLJ54370; FLJ54392; FLJ54408; FLJ75127; Heat shock 70 kDa protein 1; Heat shock 70 kDa protein 1/2; Heat shock 70 kDa protein 1A/1B; Heat shock 70kDa protein 1A; Heat shock 70kDa protein 1B; Heat shock induced protein; HEL S 103; HSP70 1; HSP70 1B; HSP70 2; HSP70-1/HSP70-2; HSP70-1A; HSP70.1; HSP70.1/HSP70.2; HSP70I; HSP71_HUMAN; HSP72; HSPA1; HSPA1A; HSPA1B;
抗原和靶标
- P0DMV8 HS71A_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MAKAAAIGIDLGTTYSCVGVFQHGKVEIIANDQGNRTTPSYVAFTDTERLIGDAAKNQVALNPQNTVFDAKRLIGRKFGDPVVQSDMKHWPFQVINDGDKPKVQVSYKGETKAFYPEEISSMVLTKMKEIAEAYLGYPVTNAVITVPAYFNDSQRQATKDAGVIAGLNVLRIINEPTAAAIAYGLDRTGKGERNVLIFDLGGGTFDVSILTIDDGIFEVKATAGDTHLGGEDFDNRLVNHFVEEFKRKHKKDISQNKRAVRRLRTACERAKRTLSSSTQASLEIDSLFEGIDFYTSITRARFEELCSDLFRSTLEPVEKALRDAKLDKAQIHDLVLVGGSTRIPKVQKLLQDFFNGRDLNKSINPDEAVAYGAAVQAAILMGDKSENVQDLLLLDVAPLSLGLETAGGVMTALIKRNSTIPTKQTQIFTTYSDNQPGVLIQVYEGERAMTKDNNLLGRFELSGIPPAPRGVPQIEVTFDIDANGILNVTATDKSTGKANKITITNDKGRLSKEEIERMVQEAEKYKAEDEVQRERVSAKNALESYAFNMKSAVEDEGLKGKISEADKKKVLDKCQEVISWLDANTLAEKDEFEHKRKELEQVCNPIISGLYQGAGGPGPGGFGAQGPKGGSGSGPTIEEVD
种属预测
score>80的预测可信度较高,可尝试用于WB检测。*预测模型主要基于免疫原序列比对,结果仅作参考,不作为质保凭据。
High(score>80) Medium(80>score>50) Low(score<50) No confidence
研究背景
Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation. Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle. Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling. Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation. Negatively regulates heat shock-induced HSF1 transcriptional activity during the attenuation and recovery phase period of the heat shock response.
(Microbial infection) In case of rotavirus A infection, serves as a post-attachment receptor for the virus to facilitate entry into the cell.
In response to cellular stress, acetylated at Lys-77 by NA110 and then gradually deacetylated by HDAC4 at later stages. Acetylation enhances its chaperone activity and also determines whether it will function as a chaperone for protein refolding or degradation by controlling its binding to co-chaperones HOPX and STUB1. The acetylated form and the non-acetylated form bind to HOPX and STUB1 respectively. Acetylation also protects cells against various types of cellular stress.
Cytoplasm. Nucleus. Cytoplasm>Cytoskeleton>Microtubule organizing center>Centrosome.
Note: Localized in cytoplasmic mRNP granules containing untranslated mRNAs.
Component of the CatSper complex. Identified in a IGF2BP1-dependent mRNP granule complex containing untranslated mRNAs. Interacts with CHCHD3, DNAJC7, IRAK1BP1, PPP5C and TSC2. Interacts with TERT; the interaction occurs in the absence of the RNA component, TERC, and dissociates once the TERT complex has formed. Interacts with TRIM5 (via B30.2/SPRY domain). Interacts with METTL21A. Interacts with DNAAF2 (By similarity). Interacts with PRKN. Interacts with FOXP3. Interacts with NOD2; the interaction enhances NOD2 stability. Interacts with DNAJC9 (via J domain). Interacts with ATF5; the interaction protects ATF5 from degradation via proteasome-dependent and caspase-dependent processes. Interacts with RNF207 (via the C-terminus); this interaction additively increases KCNH2 expression. Interacts with HSF1 (via transactivation domain); this interaction results in the inhibition of heat shock- and HSF1-induced transcriptional activity during the attenuation and recovery phase period of the heat shock response. Interacts with NAA10, HSP40, HSP90 and HDAC4. The acetylated form and the non-acetylated form interact with HOPX and STUB1 respectively. Interacts with NEDD1. Interacts (via NBD) with BAG1, BAG2, BAG3 and HSPH1/HSP105. Interacts with SMAD3. Interacts with DNAJC8. Interacts with NLRP12.
The N-terminal nucleotide binding domain (NBD) (also known as the ATPase domain) is responsible for binding and hydrolyzing ATP. The C-terminal substrate-binding domain (SBD) (also known as peptide-binding domain) binds to the client/substrate proteins. The two domains are allosterically coupled so that, when ATP is bound to the NBD, the SBD binds relatively weakly to clients. When ADP is bound in the NBD, a conformational change enhances the affinity of the SBD for client proteins.
Belongs to the heat shock protein 70 family.
研究领域
· Cellular Processes > Transport and catabolism > Endocytosis. (View pathway)
· Environmental Information Processing > Signal transduction > MAPK signaling pathway. (View pathway)
· Genetic Information Processing > Transcription > Spliceosome.
· Genetic Information Processing > Folding, sorting and degradation > Protein processing in endoplasmic reticulum. (View pathway)
· Human Diseases > Neurodegenerative diseases > Prion diseases.
· Human Diseases > Infectious diseases: Bacterial > Legionellosis.
· Human Diseases > Infectious diseases: Parasitic > Toxoplasmosis.
· Human Diseases > Infectious diseases: Viral > Measles.
· Human Diseases > Infectious diseases: Viral > Influenza A.
· Human Diseases > Infectious diseases: Viral > Epstein-Barr virus infection.
· Organismal Systems > Aging > Longevity regulating pathway - multiple species. (View pathway)
· Organismal Systems > Immune system > Antigen processing and presentation. (View pathway)
· Organismal Systems > Endocrine system > Estrogen signaling pathway. (View pathway)
文献引用
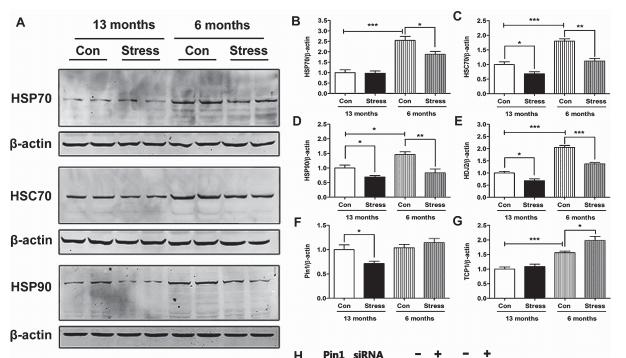
Application: WB Species: Mouse Sample:
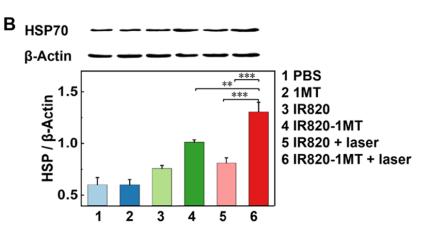
Application: WB Species: mouse Sample: B16F10 cells
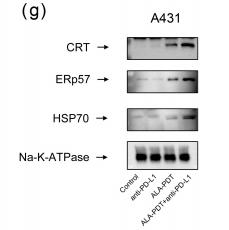
Application: WB Species: human Sample: TNBC cells
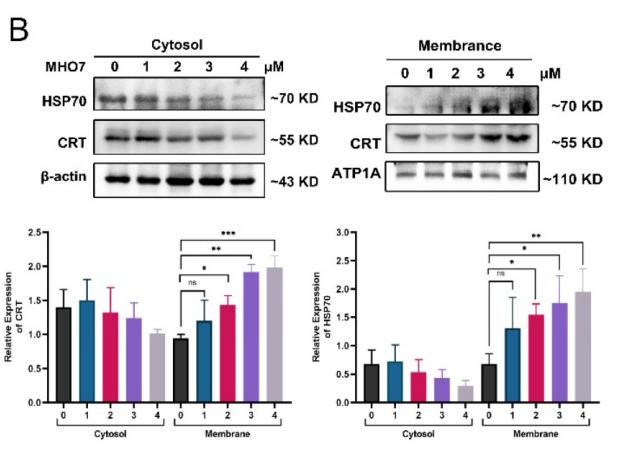
Application: WB Species: mouse Sample: tumor
限制条款
产品的规格、报价、验证数据请以官网为准,官网链接:www.affbiotech.com | www.affbiotech.cn(简体中文)| www.affbiotech.jp(日本語)产品的数据信息为Affinity所有,未经授权不得收集Affinity官网数据或资料用于商业用途,对抄袭产品数据的行为我们将保留诉诸法律的权利。
产品相关数据会因产品批次、产品检测情况随时调整,如您已订购该产品,请以订购时随货说明书为准,否则请以官网内容为准,官网内容有改动时恕不另行通知。
Affinity保证所销售产品均经过严格质量检测。如您购买的商品在规定时间内出现问题需要售后时,请您在Affinity官方渠道提交售后申请。产品仅供科学研究使用。不用于诊断和治疗。
产品未经授权不得转售。
Affinity Biosciences将不会对在使用我们的产品时可能发生的专利侵权或其他侵权行为负责。Affinity Biosciences, Affinity Biosciences标志和所有其他商标所有权归Affinity Biosciences LTD.