产品描述
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: 适用于变性蛋白样本的免疫印迹检测. IHC: 适用于组织样本的石蜡(IHC-p)或冰冻(IHC-f)切片样本的免疫组化/荧光检测. IF/ICC: 适用于细胞样本的荧光检测. ELISA(peptide): 适用于抗原肽的ELISA检测.
引用格式: Affinity Biosciences Cat# DF6421, RRID:AB_2838384.
展开/折叠
40; AAG 4; AAG4; Aging associated protein 4; Aging-associated gene 4 protein; AI893575; APO J; Apo-J; APOJ; ApoJalpha; ApoJbeta; Apolipoprotein J; ApolipoproteinJ; CLI; CLU; CLU1; CLU2; CLUS_HUMAN; Clusterin alpha chain; Clusterin; Clusterin beta chain; Complement associated protein SP 40 40; Complement associated protein SP 40; Complement associated protein SP40; Complement cytolysis inhibitor a chain; Complement cytolysis inhibitor; Complement cytolysis inhibitor b chain; Complement lysis inhibitor; Complement-associated protein SP-40; D14Ucla3; Dimeric acid glycoprotein; Glycoprotein 80; Glycoprotein III; GP80; Ku70-binding protein 1; KUB 1; KUB1; MGC24903; NA1/NA2; RATTRPM2B; SGP 2; SGP2; SP 40; SP40; Sugp-2; Sulfated glycoprotein 2; Testosterone repressed prostate message 2; Testosterone-repressed prostate message 2; TRPM 2; TRPM-2; TRPM2; TRPM2B; Trpmb;
抗原和靶标
A synthesized peptide derived from human CLU, corresponding to a region within the internal amino acids.
Detected in blood plasma, cerebrospinal fluid, milk, seminal plasma and colon mucosa. Detected in the germinal center of colon lymphoid nodules and in colon parasympathetic ganglia of the Auerbach plexus (at protein level). Ubiquitous. Detected in brain, testis, ovary, liver and pancreas, and at lower levels in kidney, heart, spleen and lung.
- P10909 CLUS_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MMKTLLLFVGLLLTWESGQVLGDQTVSDNELQEMSNQGSKYVNKEIQNAVNGVKQIKTLIEKTNEERKTLLSNLEEAKKKKEDALNETRESETKLKELPGVCNETMMALWEECKPCLKQTCMKFYARVCRSGSGLVGRQLEEFLNQSSPFYFWMNGDRIDSLLENDRQQTHMLDVMQDHFSRASSIIDELFQDRFFTREPQDTYHYLPFSLPHRRPHFFFPKSRIVRSLMPFSPYEPLNFHAMFQPFLEMIHEAQQAMDIHFHSPAFQHPPTEFIREGDDDRTVCREIRHNSTGCLRMKDQCDKCREILSVDCSTNNPSQAKLRRELDESLQVAERLTRKYNELLKSYQWKMLNTSSLLEQLNEQFNWVSRLANLTQGEDQYYLRVTTVASHTSDSDVPSGVTEVVVKLFDSDPITVTVPVEVSRKNPKFMETVAEKALQEYRKKHREE
研究背景
Functions as extracellular chaperone that prevents aggregation of non native proteins. Prevents stress-induced aggregation of blood plasma proteins. Inhibits formation of amyloid fibrils by APP, APOC2, B2M, CALCA, CSN3, SNCA and aggregation-prone LYZ variants (in vitro). Does not require ATP. Maintains partially unfolded proteins in a state appropriate for subsequent refolding by other chaperones, such as HSPA8/HSC70. Does not refold proteins by itself. Binding to cell surface receptors triggers internalization of the chaperone-client complex and subsequent lysosomal or proteasomal degradation. Protects cells against apoptosis and against cytolysis by complement. Intracellular forms interact with ubiquitin and SCF (SKP1-CUL1-F-box protein) E3 ubiquitin-protein ligase complexes and promote the ubiquitination and subsequent proteasomal degradation of target proteins. Promotes proteasomal degradation of COMMD1 and IKBKB. Modulates NF-kappa-B transcriptional activity. A mitochondrial form suppresses BAX-dependent release of cytochrome c into the cytoplasm and inhibit apoptosis. Plays a role in the regulation of cell proliferation. An intracellular form suppresses stress-induced apoptosis by stabilizing mitochondrial membrane integrity through interaction with HSPA5. Secreted form does not affect caspase or BAX-mediated intrinsic apoptosis and TNF-induced NF-kappa-B-activity. Secreted form act as an important modulator during neuronal differentiation through interaction with STMN3 (By similarity). Plays a role in the clearance of immune complexes that arise during cell injury (By similarity).
Does not affect caspase or BAX-mediated intrinsic apoptosis and TNF-induced NF-kappa-B-activity.
Does not affect caspase or BAX-mediated intrinsic apoptosis and TNF-induced NF-kappa-B-activity. Promotes cell death through interaction with BCL2L1 that releases and activates BAX.
Proteolytically cleaved on its way through the secretory system, probably within the Golgi lumen. Proteolytic cleavage is not necessary for its chaperone activity. All non-secreted forms are not proteolytically cleaved. Chaperone activity of uncleaved forms is dependent on a non-reducing envoronment.
Polyubiquitinated, leading to proteasomal degradation. Under cellular stress, the intracellular level of cleaved form is reduced due to proteasomal degradation.
Extensively glycosylated with sulfated N-linked carbohydrates. About 30% of the protein mass is comprised of complex N-linked carbohydrate. Endoplasmic reticulum (ER) stress induces changes in glycosylation status and increases level of hypoglycosylated forms. Core carbohydrates are essential for chaperone activity. Non-secreted forms are hypoglycosylated or unglycosylated.
Secreted.
Note: Can retrotranslocate from the secretory compartments to the cytosol upon cellular stress.
Cytoplasm.
Note: Keeps cytoplasmic localization in stressed and unstressed cell.
Cytoplasm.
Note: Keeps cytoplasmic localization in stressed and unstressed cell.
Nucleus. Cytoplasm. Mitochondrion membrane>Peripheral membrane protein>Cytoplasmic side. Cytoplasm>Cytosol. Microsome. Endoplasmic reticulum. Mitochondrion. Mitochondrion membrane. Cytoplasm>Perinuclear region. Cytoplasmic vesicle>Secretory vesicle>Chromaffin granule.
Note: Secreted isoforms can retrotranslocate from the secretory compartments to the cytosol upon cellular stress (PubMed:17451556). Detected in perinuclear foci that may be aggresomes containing misfolded, ubiquitinated proteins (PubMed:20068069). Detected at the mitochondrion membrane upon induction of apoptosis (PubMed:17689225). Under ER stress, a immaturely glycosylated pre-secreted form retrotranslocates from the endoplasmic reticulum (ER)-Golgi network to the cytoplasm to localize in the mitochondria through HSPA5 interaction (PubMed:22689054). ER stress reduces secretion (PubMed:22689054). Under the stress, minor amounts of non-secreted forms accumulate in cytoplasm (PubMed:24073260, PubMed:22689054, PubMed:17451556). Non-secreted forms emerge mainly from failed translocation, alternative splicing or non-canonical initiation start codon (PubMed:24073260, PubMed:12551933).
Detected in blood plasma, cerebrospinal fluid, milk, seminal plasma and colon mucosa. Detected in the germinal center of colon lymphoid nodules and in colon parasympathetic ganglia of the Auerbach plexus (at protein level). Ubiquitous. Detected in brain, testis, ovary, liver and pancreas, and at lower levels in kidney, heart, spleen and lung.
Belongs to the clusterin family.
研究领域
· Organismal Systems > Immune system > Complement and coagulation cascades. (View pathway)
文献引用
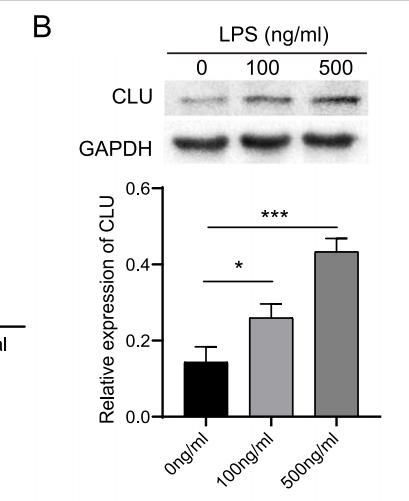
Application: WB Species: Human Sample: TEV-1 cells
限制条款
产品的规格、报价、验证数据请以官网为准,官网链接:www.affbiotech.com | www.affbiotech.cn(简体中文)| www.affbiotech.jp(日本語)产品的数据信息为Affinity所有,未经授权不得收集Affinity官网数据或资料用于商业用途,对抄袭产品数据的行为我们将保留诉诸法律的权利。
产品相关数据会因产品批次、产品检测情况随时调整,如您已订购该产品,请以订购时随货说明书为准,否则请以官网内容为准,官网内容有改动时恕不另行通知。
Affinity保证所销售产品均经过严格质量检测。如您购买的商品在规定时间内出现问题需要售后时,请您在Affinity官方渠道提交售后申请。产品仅供科学研究使用。不用于诊断和治疗。
产品未经授权不得转售。
Affinity Biosciences将不会对在使用我们的产品时可能发生的专利侵权或其他侵权行为负责。Affinity Biosciences, Affinity Biosciences标志和所有其他商标所有权归Affinity Biosciences LTD.