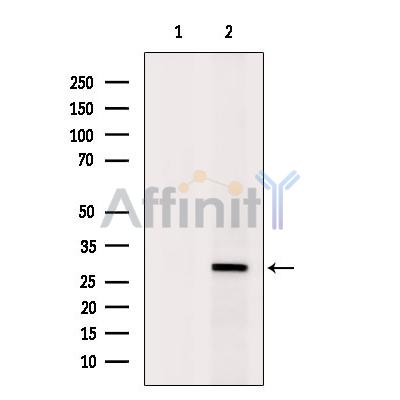
产品描述
*The optimal dilutions should be determined by the end user.
*Tips:
WB: 适用于变性蛋白样本的免疫印迹检测. IHC: 适用于组织样本的石蜡(IHC-p)或冰冻(IHC-f)切片样本的免疫组化/荧光检测. IF/ICC: 适用于细胞样本的荧光检测. ELISA(peptide): 适用于抗原肽的ELISA检测.
引用格式: Affinity Biosciences Cat# DF6529, RRID:AB_2838491.
展开/折叠
Alternative splicing factor 1; Alternative-splicing factor 1; arginine/serine-rich 1; ASF 1; ASF; ASF-1; ASF1; FLJ53078; MGC5228; P33 subunit; Pre mRNA splicing factor SF2 P33 subunit; pre-mRNA-splicing factor SF2; Serine/arginine-rich splicing factor 1; SF2; SF2P33; SFRS1; Splicing factor 2 alternate splicing factor; Splicing factor 2; Splicing factor; Splicing factor arginine/serine rich 1; SR Splicing factor 1; SRp30a; srsf1; SRSF1_HUMAN;
抗原和靶标
- Q07955 SRSF1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MSGGGVIRGPAGNNDCRIYVGNLPPDIRTKDIEDVFYKYGAIRDIDLKNRRGGPPFAFVEFEDPRDAEDAVYGRDGYDYDGYRLRVEFPRSGRGTGRGGGGGGGGGAPRGRYGPPSRRSENRVVVSGLPPSGSWQDLKDHMREAGDVCYADVYRDGTGVVEFVRKEDMTYAVRKLDNTKFRSHEGETAYIRVKVDGPRSPSYGRSRSRSRSRSRSRSRSNSRSRSYSPRRSRGSPRYSPRHSRSRSRT
种属预测
score>80的预测可信度较高,可尝试用于WB检测。*预测模型主要基于免疫原序列比对,结果仅作参考,不作为质保凭据。
High(score>80) Medium(80>score>50) Low(score<50) No confidence
研究背景
Plays a role in preventing exon skipping, ensuring the accuracy of splicing and regulating alternative splicing. Interacts with other spliceosomal components, via the RS domains, to form a bridge between the 5'- and 3'-splice site binding components, U1 snRNP and U2AF. Can stimulate binding of U1 snRNP to a 5'-splice site-containing pre-mRNA. Binds to purine-rich RNA sequences, either the octamer, 5'-RGAAGAAC-3' (r=A or G) or the decamers, AGGACAGAGC/AGGACGAAGC. Binds preferentially to the 5'-CGAGGCG-3' motif in vitro. Three copies of the octamer constitute a powerful splicing enhancer in vitro, the ASF/SF2 splicing enhancer (ASE) which can specifically activate ASE-dependent splicing. Isoform ASF-2 and isoform ASF-3 act as splicing repressors. May function as export adapter involved in mRNA nuclear export through the TAP/NXF1 pathway.
Phosphorylated by CLK1, CLK2, CLK3 and CLK4. Phosphorylated by SRPK1 at multiple serines in its RS domain via a directional (C-terminal to N-terminal) and a dual-track mechanism incorporating both processive phosphorylation (in which the kinase stays attached to the substrate after each round of phosphorylation) and distributive phosphorylation steps (in which the kinase and substrate dissociate after each phosphorylation event). The RS domain of SRSF1 binds to a docking groove in the large lobe of the kinase domain of SRPK1 and this induces certain structural changes in SRPK1 and/or RRM 2 domain of SRSF1, allowing RRM 2 to bind the kinase and initiate phosphorylation. The cycles continue for several phosphorylation steps in a processive manner (steps 1-8) until the last few phosphorylation steps (approximately steps 9-12). During that time, a mechanical stress induces the unfolding of the beta-4 motif in RRM 2, which then docks at the docking groove of SRPK1. This also signals RRM 2 to begin to dissociate, which facilitates SRSF1 dissociation after phosphorylation is completed.
Asymmetrically dimethylated at arginines, probably by PRMT1, methylation promotes localization to nuclear speckles.
Cytoplasm. Nucleus speckle.
Note: In nuclear speckles. Shuttles between the nucleus and the cytoplasm (PubMed:12215544, PubMed:20308322, PubMed:9420331, PubMed:24449914). Nuclear import is mediated via interaction with TNPO3 (PubMed:24449914).
Consists of two polypeptides of p32 and p33. In vitro, self-associates and binds SRSF2, SNRNP70 and U2AF1 but not U2AF2. Binds SREK1/SFRS12. Interacts with SAFB/SAFB1. Interacts with PSIP1/LEDGF. Interacts with SRPK1. Identified in the spliceosome C complex. Interacts with RSRC1 (via Arg/Ser-rich domain). Interacts with ZRSR2/U2AF1-RS2. Interacts with CCDC55 (via C-terminus). Interacts with SRPK1 and a sliding docking interaction is essential for its sequential and processive phosphorylation by SRPK1. Interacts with NXF1. Interacts with CCNL1, CCNL2 and CDK11B. Interacts with RRP1B. Interacts (when phosphorylated in its RS domain) with TNPO3; promoting nuclear import.
The RRM 2 domain plays an important role in governing both the binding mode and the phosphorylation mechanism of the RS domain by SRPK1. RS domain and RRM 2 are uniquely positioned to initiate a highly directional (C-terminus to N-terminus) phosphorylation reaction in which the RS domain slides through an extended electronegative channel separating the docking groove of SRPK1 and the active site. RRM 2 binds toward the periphery of the active site and guides the directional phosphorylation mechanism. Both the RS domain and an RRM domain are required for nucleocytoplasmic shuttling.
Belongs to the splicing factor SR family.
研究领域
· Genetic Information Processing > Transcription > Spliceosome.
· Human Diseases > Infectious diseases: Viral > Herpes simplex infection.
· Organismal Systems > Immune system > IL-17 signaling pathway. (View pathway)
限制条款
产品的规格、报价、验证数据请以官网为准,官网链接:www.affbiotech.com | www.affbiotech.cn(简体中文)| www.affbiotech.jp(日本語)产品的数据信息为Affinity所有,未经授权不得收集Affinity官网数据或资料用于商业用途,对抄袭产品数据的行为我们将保留诉诸法律的权利。
产品相关数据会因产品批次、产品检测情况随时调整,如您已订购该产品,请以订购时随货说明书为准,否则请以官网内容为准,官网内容有改动时恕不另行通知。
Affinity保证所销售产品均经过严格质量检测。如您购买的商品在规定时间内出现问题需要售后时,请您在Affinity官方渠道提交售后申请。产品仅供科学研究使用。不用于诊断和治疗。
产品未经授权不得转售。
Affinity Biosciences将不会对在使用我们的产品时可能发生的专利侵权或其他侵权行为负责。Affinity Biosciences, Affinity Biosciences标志和所有其他商标所有权归Affinity Biosciences LTD.